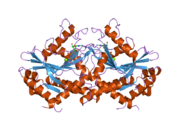Protein-coding gene in the species Homo sapiens
| GBP1 |
|---|
 |
| Available structures |
|---|
| PDB | Human UniProt search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
1DG3, 1F5N, 2B8W, 2B92, 2BC9, 2D4H |
|
|
| Identifiers |
|---|
| Aliases | GBP1, guanylate binding protein 1 |
|---|
| External IDs | OMIM: 600411; HomoloGene: 133759; GeneCards: GBP1; OMA:GBP1 - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 1 (human)[1] |
|---|
| | Band | 1p22.2 | Start | 89,051,882 bp[1] |
|---|
| End | 89,065,360 bp[1] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - pericardium
- trigeminal ganglion
- mucosa of paranasal sinus
- lower lobe of lung
- superficial temporal artery
- appendix
- gallbladder
- monocyte
- tail of epididymis
- epithelium of nasopharynx
|
| | | More reference expression data |
|
|---|
| BioGPS | 
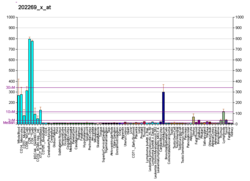 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - nucleotide binding
- Hsp90 protein binding
- spectrin binding
- protein binding
- identical protein binding
- enzyme binding
- actin binding
- hydrolase activity
- cytokine binding
- GTPase activity
- GTP binding
- GDP binding
- protein homodimerization activity
| | Cellular component | - cytosol
- membrane
- Golgi membrane
- plasma membrane
- extracellular region
- cytoplasm
- Golgi apparatus
- vesicle membrane
- actin cytoskeleton
- cytoplasmic vesicle
| | Biological process | - immune system process
- interferon-gamma-mediated signaling pathway
- negative regulation of T cell receptor signaling pathway
- negative regulation of protein localization to plasma membrane
- defense response to virus
- regulation of calcium-mediated signaling
- negative regulation of ERK1 and ERK2 cascade
- negative regulation of substrate adhesion-dependent cell spreading
- regulation of protein localization to plasma membrane
- protein homooligomerization
- cellular response to interferon-gamma
- cellular response to interleukin-1
- cellular response to tumor necrosis factor
- protein localization to vacuole
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | | |
|---|
| RefSeq (protein) | | |
|---|
| Location (UCSC) | Chr 1: 89.05 – 89.07 Mb | n/a |
|---|
| PubMed search | [2] | n/a |
|---|
|
| Wikidata |
|
Interferon-induced guanylate-binding protein 1 is a protein that in humans is encoded by the GBP1 gene.[3][4] It belongs to the dynamin superfamily of large GTPases.[5]
Function
Guanylate binding protein expression is induced by interferon. Guanylate binding proteins are characterized by their ability to specifically bind guanine nucleotides (GMP, GDP, and GTP) and are distinguished from the GTP-binding proteins by the presence of 2 binding motifs rather than 3.[4]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000117228 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Strehlow I, Lohmann-Matthes ML, Decker T (Aug 1994). "The interferon-inducible GBP1 gene: structure and mapping to human chromosome 1". Gene. 144 (2): 295–9. doi:10.1016/0378-1119(94)90393-X. PMID 7518790.
- ^ a b "Entrez Gene: GBP1 guanylate binding protein 1, interferon-inducible, 67kDa".
- ^ Praefcke GJ, McMahon HT (February 2004). "The dynamin superfamily: universal membrane tubulation and fission molecules?". Nat. Rev. Mol. Cell Biol. 5 (2): 133–47. doi:10.1038/nrm1313. PMID 15040446. S2CID 6305282.
Further reading
- Anderson NL, Anderson NG (2003). "The human plasma proteome: history, character, and diagnostic prospects". Mol. Cell. Proteomics. 1 (11): 845–67. doi:10.1074/mcp.R200007-MCP200. PMID 12488461.
- Naschberger E, Bauer M, Stürzl M (2006). "Human guanylate binding protein-1 (hGBP-1) characterizes and establishes a non-angiogenic endothelial cell activation phenotype in inflammatory diseases". Adv. Enzyme Regul. 45: 215–27. doi:10.1016/j.advenzreg.2005.02.011. PMID 16005050.
- Cheng YS, Patterson CE, Staeheli P (1991). "Interferon-induced guanylate-binding proteins lack an N(T)KXD consensus motif and bind GMP in addition to GDP and GTP". Mol. Cell. Biol. 11 (9): 4717–25. doi:10.1128/MCB.11.9.4717. PMC 361367. PMID 1715024.
- Nantais DE, Schwemmle M, Stickney JT, et al. (1996). "Prenylation of an interferon-gamma-induced GTP-binding protein: the human guanylate binding protein, huGBP1". J. Leukoc. Biol. 60 (3): 423–31. doi:10.1002/jlb.60.3.423. PMID 8830800. S2CID 33727864.
- Prakash B, Praefcke GJ, Renault L, et al. (2000). "Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins". Nature. 403 (6769): 567–71. Bibcode:2000Natur.403..567P. doi:10.1038/35000617. PMID 10676968. S2CID 4431592.
- Kumar S, Li Q, Dua A, et al. (2001). "Messenger ribonucleic acid encoding interferon-inducible guanylate binding protein 1 is induced in human endometrium within the putative window of implantation". J. Clin. Endocrinol. Metab. 86 (6): 2420–7. doi:10.1210/jcem.86.6.7534. PMID 11397834.
- Guenzi E, Töpolt K, Cornali E, et al. (2001). "The helical domain of GBP-1 mediates the inhibition of endothelial cell proliferation by inflammatory cytokines". EMBO J. 20 (20): 5568–77. doi:10.1093/emboj/20.20.5568. PMC 125279. PMID 11598000.
- Lubeseder-Martellato C, Guenzi E, Jörg A, et al. (2002). "Guanylate-Binding Protein-1 Expression Is Selectively Induced by Inflammatory Cytokines and Is an Activation Marker of Endothelial Cells during Inflammatory Diseases". Am. J. Pathol. 161 (5): 1749–59. doi:10.1016/S0002-9440(10)64452-5. PMC 1850787. PMID 12414522.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Adkins JN, Varnum SM, Auberry KJ, et al. (2003). "Toward a human blood serum proteome: analysis by multidimensional separation coupled with mass spectrometry". Mol. Cell. Proteomics. 1 (12): 947–55. doi:10.1074/mcp.M200066-MCP200. PMID 12543931.
- Guenzi E, Töpolt K, Lubeseder-Martellato C, et al. (2003). "The guanylate binding protein-1 GTPase controls the invasive and angiogenic capability of endothelial cells through inhibition of MMP-1 expression". EMBO J. 22 (15): 3772–82. doi:10.1093/emboj/cdg382. PMC 169055. PMID 12881412.
- Naschberger E, Werner T, Vicente AB, et al. (2004). "Nuclear factor-kappaB motif and interferon-alpha-stimulated response element co-operate in the activation of guanylate-binding protein-1 expression by inflammatory cytokines in endothelial cells". Biochem. J. 379 (Pt 2): 409–20. doi:10.1042/BJ20031873. PMC 1224089. PMID 14741045.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The Status, Quality, and Expansion of the NIH Full-Length cDNA Project: The Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Praefcke GJ, Kloep S, Benscheid U, et al. (2004). "Identification of residues in the human guanylate-binding protein 1 critical for nucleotide binding and cooperative GTP hydrolysis". J. Mol. Biol. 344 (1): 257–69. doi:10.1016/j.jmb.2004.09.026. PMID 15504415.
- Modiano N, Lu YE, Cresswell P (2005). "Golgi targeting of human guanylate-binding protein-1 requires nucleotide binding, isoprenylation, and an IFN-γ-inducible cofactor". Proc. Natl. Acad. Sci. U.S.A. 102 (24): 8680–5. Bibcode:2005PNAS..102.8680M. doi:10.1073/pnas.0503227102. PMC 1150846. PMID 15937107.
- Ghosh A, Praefcke GJ, Renault L, et al. (2006). "How guanylate-binding proteins achieve assembly-stimulated processive cleavage of GTP to GMP". Nature. 440 (7080): 101–4. Bibcode:2006Natur.440..101G. doi:10.1038/nature04510. PMID 16511497. S2CID 4405773.
PDB gallery
-
1dg3: STRUCTURE OF HUMAN GUANYLATE BINDING PROTEIN-1 IN NUCLEOTIDE FREE FORM -
1f5n: HUMAN GUANYLATE BINDING PROTEIN-1 IN COMPLEX WITH THE GTP ANALOGUE, GMPPNP. -
2b8w: Crystal-structure of the N-terminal Large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with GMP/AlF4 -
2b92: Crystal-structure of the N-terminal Large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with GDP/AlF3 -
2bc9: Crystal-structure of the N-terminal large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with non-hydrolysable GTP analogue GppNHp -
2d4h: Crystal-structure of the N-terminal large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with GMP |
 | This article on a gene on human chromosome 1 is a stub. You can help Wikipedia by expanding it. |

 1dg3: STRUCTURE OF HUMAN GUANYLATE BINDING PROTEIN-1 IN NUCLEOTIDE FREE FORM
1dg3: STRUCTURE OF HUMAN GUANYLATE BINDING PROTEIN-1 IN NUCLEOTIDE FREE FORM 1f5n: HUMAN GUANYLATE BINDING PROTEIN-1 IN COMPLEX WITH THE GTP ANALOGUE, GMPPNP.
1f5n: HUMAN GUANYLATE BINDING PROTEIN-1 IN COMPLEX WITH THE GTP ANALOGUE, GMPPNP. 2b8w: Crystal-structure of the N-terminal Large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with GMP/AlF4
2b8w: Crystal-structure of the N-terminal Large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with GMP/AlF4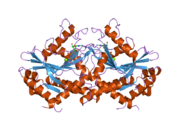 2b92: Crystal-structure of the N-terminal Large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with GDP/AlF3
2b92: Crystal-structure of the N-terminal Large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with GDP/AlF3 2bc9: Crystal-structure of the N-terminal large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with non-hydrolysable GTP analogue GppNHp
2bc9: Crystal-structure of the N-terminal large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with non-hydrolysable GTP analogue GppNHp 2d4h: Crystal-structure of the N-terminal large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with GMP
2d4h: Crystal-structure of the N-terminal large GTPase Domain of human Guanylate Binding protein 1 (hGBP1) in complex with GMP