RABGEF1
| RABGEF1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | RABGEF1, RABEX5, RAP1, rabex-5, RAB guanine nucleotide exchange factor 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 609700; MGI: 1929459; HomoloGene: 8720; GeneCards: RABGEF1; OMA:RABGEF1 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Rab5 GDP/GTP exchange factor is a protein that in humans is encoded by the RABGEF1 gene.[5][6][7]
RABGEF1 forms a complex with rabaptin-5 (RABPT5; MIM 603616) that is required for endocytic membrane fusion, and it serves as a specific guanine nucleotide exchange factor for RAB5(RAB5A; MIM 179512) (Horiuchi et al., 1997) [supplied by OMIM].[7]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000154710 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000025340 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Mattera R, Arighi CN, Lodge R, Zerial M, Bonifacino JS (Dec 2002). "Divalent interaction of the GGAs with the Rabaptin-5-Rabex-5 complex". EMBO J. 22 (1): 78–88. doi:10.1093/emboj/cdg015. PMC 140067. PMID 12505986.
- ^ Nimmrich I, Erdmann S, Melchers U, Finke U, Hentsch S, Moyer MP, Hoffmann I, Muller O (Dec 2000). "Seven genes that are differentially transcribed in colorectal tumor cell lines". Cancer Lett. 160 (1): 37–43. doi:10.1016/S0304-3835(00)00553-X. PMID 11098082.
- ^ a b "Entrez Gene: RABGEF1 RAB guanine nucleotide exchange factor (GEF) 1".
Further reading
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Horiuchi H, Lippé R, McBride HM, et al. (1997). "A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function". Cell. 90 (6): 1149–59. doi:10.1016/S0092-8674(00)80380-3. PMID 9323142. S2CID 13972726.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Lippé R, Miaczynska M, Rybin V, et al. (2001). "Functional synergy between Rab5 effector Rabaptin-5 and exchange factor Rabex-5 when physically associated in a complex". Mol. Biol. Cell. 12 (7): 2219–28. doi:10.1091/mbc.12.7.2219. PMC 55678. PMID 11452015.
- de Renzis S, Sönnichsen B, Zerial M (2002). "Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes". Nat. Cell Biol. 4 (2): 124–33. doi:10.1038/ncb744. PMID 11788822. S2CID 6596498.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Hillier LW, Fulton RS, Fulton LA, et al. (2003). "The DNA sequence of human chromosome 7". Nature. 424 (6945): 157–64. Bibcode:2003Natur.424..157H. doi:10.1038/nature01782. PMID 12853948.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Jin J, Smith FD, Stark C, et al. (2004). "Proteomic, functional, and domain-based analysis of in vivo 14-3-3 binding proteins involved in cytoskeletal regulation and cellular organization". Curr. Biol. 14 (16): 1436–50. Bibcode:2004CBio...14.1436J. doi:10.1016/j.cub.2004.07.051. PMID 15324660. S2CID 2371325.
- Delprato A, Merithew E, Lambright DG (2004). "Structure, exchange determinants, and family-wide rab specificity of the tandem helical bundle and Vps9 domains of Rabex-5". Cell. 118 (5): 607–17. doi:10.1016/j.cell.2004.08.009. PMID 15339665. S2CID 9638315.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Rual JF, Venkatesan K, Hao T, et al. (2005). "Towards a proteome-scale map of the human protein-protein interaction network". Nature. 437 (7062): 1173–8. Bibcode:2005Natur.437.1173R. doi:10.1038/nature04209. PMID 16189514. S2CID 4427026.
- Kimura K, Wakamatsu A, Suzuki Y, et al. (2006). "Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes". Genome Res. 16 (1): 55–65. doi:10.1101/gr.4039406. PMC 1356129. PMID 16344560.
- Penengo L, Mapelli M, Murachelli AG, et al. (2006). "Crystal structure of the ubiquitin binding domains of rabex-5 reveals two modes of interaction with ubiquitin". Cell. 124 (6): 1183–95. doi:10.1016/j.cell.2006.02.020. PMID 16499958. S2CID 2639312.
- Kalesnikoff J, Rios EJ, Chen CC, et al. (2007). "Roles of RabGEF1/Rabex-5 domains in regulating Fc epsilon RI surface expression and Fc epsilon RI-dependent responses in mast cells". Blood. 109 (12): 5308–17. doi:10.1182/blood-2007-01-067363. PMC 1890836. PMID 17341663.
- Ewing RM, Chu P, Elisma F, et al. (2007). "Large-scale mapping of human protein-protein interactions by mass spectrometry". Mol. Syst. Biol. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948. PMID 17353931.
- Delprato A, Lambright DG (2007). "Structural basis for Rab GTPase activation by VPS9 domain exchange factors". Nat. Struct. Mol. Biol. 14 (5): 406–12. doi:10.1038/nsmb1232. PMC 2254184. PMID 17450153.
- v
- t
- e
-
 1txu: Crystal Structure of the Vps9 Domain of Rabex-5
1txu: Crystal Structure of the Vps9 Domain of Rabex-5 -
 2c7m: HUMAN RABEX-5 RESIDUES 1-74 IN COMPLEX WITH UBIQUITIN
2c7m: HUMAN RABEX-5 RESIDUES 1-74 IN COMPLEX WITH UBIQUITIN -
 2c7n: HUMAN RABEX-5 RESIDUES 1-74 IN COMPLEX WITH UBIQUITIN
2c7n: HUMAN RABEX-5 RESIDUES 1-74 IN COMPLEX WITH UBIQUITIN -
 2fid: Crystal Structure of a Bovine Rabex-5 fragment complexed with ubiquitin
2fid: Crystal Structure of a Bovine Rabex-5 fragment complexed with ubiquitin -
 2fif: Crystal Structure of a Bovine Rabex-5 fragment complexed with ubiquitin
2fif: Crystal Structure of a Bovine Rabex-5 fragment complexed with ubiquitin -
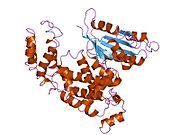 2ot3: Crystal structure of rabex-5 VPS9 domain in complex with nucleotide free RAB21
2ot3: Crystal structure of rabex-5 VPS9 domain in complex with nucleotide free RAB21
 | This protein-related article is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e























