Protein-coding gene in the species Homo sapiens
| UBE4B |
|---|
 |
| Available structures |
|---|
| PDB | Ortholog search: PDBe RCSB |
|---|
| List of PDB id codes |
|---|
2KRE, 3L1X, 3L1Z |
|
|
| Identifiers |
|---|
| Aliases | UBE4B, E4, HDNB1, UBOX3, UFD2, UFD2A, ubiquitination factor E4B |
|---|
| External IDs | OMIM: 613565; MGI: 1927086; HomoloGene: 107623; GeneCards: UBE4B; OMA:UBE4B - orthologs |
|---|
| Gene location (Human) |
|---|
 | | Chr. | Chromosome 1 (human)[1] |
|---|
| | Band | 1p36.22 | Start | 10,032,832 bp[1] |
|---|
| End | 10,181,239 bp[1] |
|---|
|
| Gene location (Mouse) |
|---|
 | | Chr. | Chromosome 4 (mouse)[2] |
|---|
| | Band | 4 E2|4 79.08 cM | Start | 149,328,416 bp[2] |
|---|
| End | 149,426,749 bp[2] |
|---|
|
| RNA expression pattern |
|---|
| Bgee | | Human | Mouse (ortholog) |
|---|
| Top expressed in | - buccal mucosa cell
- skin of thigh
- hair follicle
- cerebellar hemisphere
- right hemisphere of cerebellum
- gastric mucosa
- gastrocnemius muscle
- inferior olivary nucleus
- Skeletal muscle tissue of rectus abdominis
- right adrenal cortex
|
| | Top expressed in | - tail of embryo
- genital tubercle
- secondary oocyte
- zygote
- internal carotid artery
- external carotid artery
- yolk sac
- facial motor nucleus
- renal corpuscle
- ureter
|
| | More reference expression data |
|
|---|
| BioGPS | 
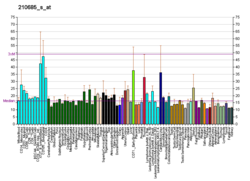
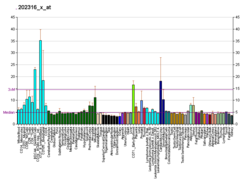 | | More reference expression data |
|
|---|
|
| Gene ontology |
|---|
| Molecular function | - ubiquitin protein ligase activity
- ATPase binding
- ubiquitin-protein transferase activity
- enzyme binding
- ATP binding
- transferase activity
- ubiquitin-ubiquitin ligase activity
- protein binding
| | Cellular component | - ubiquitin ligase complex
- nucleus
- cytoplasm
| | Biological process | - protein monoubiquitination
- response to endoplasmic reticulum stress
- ventricular trabecula myocardium morphogenesis
- protein ubiquitination
- neuron projection development
- granzyme-mediated apoptotic signaling pathway
- response to UV
- proteasome-mediated ubiquitin-dependent protein catabolic process
- protein autoubiquitination
- protein polyubiquitination
- ubiquitin-dependent ERAD pathway
- ubiquitin-dependent protein catabolic process
| | Sources:Amigo / QuickGO |
|
| Orthologs |
|---|
| Species | Human | Mouse |
|---|
| Entrez | | |
|---|
| Ensembl | | |
|---|
| UniProt | | |
|---|
| RefSeq (mRNA) | | |
|---|
| RefSeq (protein) | | |
|---|
NP_071305
NP_001391797
NP_001391798 |
|
|---|
| Location (UCSC) | Chr 1: 10.03 – 10.18 Mb | Chr 4: 149.33 – 149.43 Mb |
|---|
| PubMed search | [3] | [4] |
|---|
|
| Wikidata |
| View/Edit Human | View/Edit Mouse |
|
Ubiquitin conjugation factor E4 B is a protein that in humans is encoded by the UBE4B gene.[5][6][7]
The modification of proteins with ubiquitin is an important cellular mechanism for targeting abnormal or short-lived proteins for degradation. Ubiquitination involves at least three classes of enzymes: ubiquitin-activating enzymes, or E1s, ubiquitin-conjugating enzymes, or E2s, and ubiquitin-protein ligases, or E3s.
This gene encodes an additional conjugation factor, E4, which is involved in multiubiquitin chain assembly. This gene is also the strongest candidate in the neuroblastoma tumor suppressor genes.[7]
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000130939 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000028960 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Ishikawa K, Nagase T, Suyama M, Miyajima N, Tanaka A, Kotani H, Nomura N, Ohara O (Dec 1998). "Prediction of the coding sequences of unidentified human genes. X. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro". DNA Res. 5 (3): 169–76. doi:10.1093/dnares/5.3.169. PMID 9734811.
- ^ Koegl M, Hoppe T, Schlenker S, Ulrich HD, Mayer TU, Jentsch S (Apr 1999). "A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly". Cell. 96 (5): 635–44. doi:10.1016/S0092-8674(00)80574-7. PMID 10089879.
- ^ a b "Entrez Gene: UBE4B ubiquitination factor E4B (UFD2 homolog, yeast)".
Further reading
- Onyango P, Lubyova B, Gardellin P, et al. (1998). "Molecular cloning and expression analysis of five novel genes in chromosome 1p36". Genomics. 50 (2): 187–98. doi:10.1006/geno.1997.5186. PMID 9653645.
- Ohira M, Kageyama H, Mihara M, et al. (2000). "Identification and characterization of a 500-kb homozygously deleted region at 1p36.2-p36.3 in a neuroblastoma cell line". Oncogene. 19 (37): 4302–7. doi:10.1038/sj.onc.1203786. PMID 10980605. S2CID 24163907.
- Mahoney JA, Odin JA, White SM, et al. (2002). "The human homologue of the yeast polyubiquitination factor Ufd2p is cleaved by caspase 6 and granzyme B during apoptosis". Biochem. J. 361 (Pt 3): 587–95. doi:10.1042/0264-6021:3610587. PMC 1222341. PMID 11802788.
- Strausberg RL, Feingold EA, Grouse LH, et al. (2003). "Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences". Proc. Natl. Acad. Sci. U.S.A. 99 (26): 16899–903. Bibcode:2002PNAS...9916899M. doi:10.1073/pnas.242603899. PMC 139241. PMID 12477932.
- Kaneko C, Hatakeyama S, Matsumoto M, et al. (2003). "Characterization of the mouse gene for the U-box-type ubiquitin ligase UFD2a". Biochem. Biophys. Res. Commun. 300 (2): 297–304. doi:10.1016/S0006-291X(02)02834-6. PMID 12504083.
- Krona C, Ejeskär K, Abel F, et al. (2003). "Screening for gene mutations in a 500 kb neuroblastoma tumor suppressor candidate region in chromosome 1p; mutation and stage-specific expression in UBE4B/UFD2". Oncogene. 22 (15): 2343–51. doi:10.1038/sj.onc.1206324. PMID 12700669. S2CID 7749013.
- Ota T, Suzuki Y, Nishikawa T, et al. (2004). "Complete sequencing and characterization of 21,243 full-length human cDNAs". Nat. Genet. 36 (1): 40–5. doi:10.1038/ng1285. PMID 14702039.
- Beausoleil SA, Jedrychowski M, Schwartz D, et al. (2004). "Large-scale characterization of HeLa cell nuclear phosphoproteins". Proc. Natl. Acad. Sci. U.S.A. 101 (33): 12130–5. Bibcode:2004PNAS..10112130B. doi:10.1073/pnas.0404720101. PMC 514446. PMID 15302935.
- Okumura F, Hatakeyama S, Matsumoto M, et al. (2005). "Functional regulation of FEZ1 by the U-box-type ubiquitin ligase E4B contributes to neuritogenesis". J. Biol. Chem. 279 (51): 53533–43. doi:10.1074/jbc.M402916200. PMID 15466860.
- Gerhard DS, Wagner L, Feingold EA, et al. (2004). "The status, quality, and expansion of the NIH full-length cDNA project: the Mammalian Gene Collection (MGC)". Genome Res. 14 (10B): 2121–7. doi:10.1101/gr.2596504. PMC 528928. PMID 15489334.
- Kimura K, Wakamatsu A, Suzuki Y, et al. (2006). "Diversification of transcriptional modulation: large-scale identification and characterization of putative alternative promoters of human genes". Genome Res. 16 (1): 55–65. doi:10.1101/gr.4039406. PMC 1356129. PMID 16344560.
- Gregory SG, Barlow KF, McLay KE, et al. (2006). "The DNA sequence and biological annotation of human chromosome 1". Nature. 441 (7091): 315–21. Bibcode:2006Natur.441..315G. doi:10.1038/nature04727. PMID 16710414.
- Spinette S, Mahoney JA, Rosen A (2006). "The MPAC domain is a novel mitotically regulated domain, removed by apoptotic protease cleavage during cell death". Biochem. Biophys. Res. Commun. 347 (4): 1103–12. doi:10.1016/j.bbrc.2006.06.194. PMID 16870146.
- Olsen JV, Blagoev B, Gnad F, et al. (2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks". Cell. 127 (3): 635–48. doi:10.1016/j.cell.2006.09.026. PMID 17081983. S2CID 7827573.
External links




















