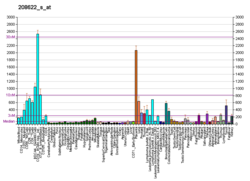
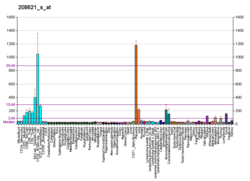
Cytovillin
| EZR | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
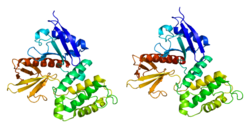 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identificadores | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Nomes alternativos | EZR, ezrin, villin-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| IDs externos | OMIM: 123900 HomoloGene: 55740 GeneCards: EZR | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Cytovillin também conhecido como ezrin ou villin-2 é uma proteína que em humanos é codificada pelo gene EZR.[2]
Estrutura
O terminal N de cytovillin contém um domínio FERM[3] que é subdividido em três subdomínios. O terminal C contém um domínio ERM.[4]
Função
A proteína periférica[5] citoplasmática codificada por esse gene pode ser fosforilada pela proteína-tirosina-quinase em microvilos e é um membro da família de proteínas ERM.[6]
Interações
O VIL2 demonstrou interagir com:
- CD43,[7]
- FASLG,[8][9]
- ICAM-1,[10]
- ICAM2,[10]
- ICAM3,[10][11]
- Merlin,[12]
- MSN,[8][13][14]
- PIK3R1,[15]
- PALLD[16]
- S100P,[17]
- SDC2,[18]
- SLC9A3R1,[19][20]
- SLC9A3R2,[21][22] and
- VCAM-1.[23]
Referências
- ↑ «Human PubMed Reference:»
- ↑ Gould KL, Bretscher A, Esch FS, Hunter T (dezembro de 1989). «cDNA cloning and sequencing of the protein-tyrosine kinase substrate, ezrin, reveals homology to band 4.1». EMBO J. 8 (13): 4133–42. PMC 401598
 . PMID 2591371. doi:10.1002/j.1460-2075.1989.tb08598.x
. PMID 2591371. doi:10.1002/j.1460-2075.1989.tb08598.x - ↑ Chishti AH, Kim AC, Marfatia SM, Lutchman M, Hanspal M, Jindal H, Liu SC, Low PS, Rouleau GA, Mohandas N, Chasis JA, Conboy JG, Gascard P, Takakuwa Y, Huang SC, Benz EJ, Bretscher A, Fehon RG, Gusella JF, Ramesh V, Solomon F, Marchesi VT, Tsukita S, Tsukita S, Hoover KB (agosto de 1998). «The FERM domain: a unique module involved in the linkage of cytoplasmic proteins to the membrane». Trends Biochem. Sci. 23 (8): 281–2. PMID 9757824. doi:10.1016/S0968-0004(98)01237-7
- ↑ Bretscher A (agosto de 1983). «Purification of an 80,000-dalton protein that is a component of the isolated microvillus cytoskeleton, and its localization in nonmuscle cells». J. Cell Biol. 97 (2): 425–32. PMC 2112519
 . PMID 6885906. doi:10.1083/jcb.97.2.425
. PMID 6885906. doi:10.1083/jcb.97.2.425 - ↑ Ghosh M, Tucker, DE., et al. (2006). «Properties of group IV phospholipase A2 family (review)». Prog. Lipid. Res. 45 (6): 487–510. PMID 16814865. doi:10.1016/j.plipres.2006.05.003
- ↑ «Entrez Gene: VIL2 villin 2 (ezrin)»
- ↑ Serrador JM, Nieto M, Alonso-Lebrero JL, del Pozo MA, Calvo J, Furthmayr H, Schwartz-Albiez R, Lozano F, González-Amaro R, Sánchez-Mateos P, Sánchez-Madrid F (junho de 1998). «CD43 interacts with moesin and ezrin and regulates its redistribution to the uropods of T lymphocytes at the cell-cell contacts». Blood. 91 (12): 4632–44. PMID 9616160
- ↑ a b Gajate C, Mollinedo F (março de 2005). «Cytoskeleton-mediated death receptor and ligand concentration in lipid rafts forms apoptosis-promoting clusters in cancer chemotherapy». J. Biol. Chem. 280 (12): 11641–7. PMID 15659383. doi:10.1074/jbc.M411781200
- ↑ Parlato S, Giammarioli AM, Logozzi M, Lozupone F, Matarrese P, Luciani F, Falchi M, Malorni W, Fais S (outubro de 2000). «CD95 (APO-1/Fas) linkage to the actin cytoskeleton through ezrin in human T lymphocytes: a novel regulatory mechanism of the CD95 apoptotic pathway». EMBO J. 19 (19): 5123–34. PMC 302100
 . PMID 11013215. doi:10.1093/emboj/19.19.5123
. PMID 11013215. doi:10.1093/emboj/19.19.5123 - ↑ a b c Heiska L, Alfthan K, Grönholm M, Vilja P, Vaheri A, Carpén O (agosto de 1998). «Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate». J. Biol. Chem. 273 (34): 21893–900. PMID 9705328. doi:10.1074/jbc.273.34.21893
- ↑ Serrador JM, Vicente-Manzanares M, Calvo J, Barreiro O, Montoya MC, Schwartz-Albiez R, Furthmayr H, Lozano F, Sánchez-Madrid F (março de 2002). «A novel serine-rich motif in the intercellular adhesion molecule 3 is critical for its ezrin/radixin/moesin-directed subcellular targeting». J. Biol. Chem. 277 (12): 10400–9. PMID 11784723. doi:10.1074/jbc.M110694200
- ↑ Grönholm M, Sainio M, Zhao F, Heiska L, Vaheri A, Carpén O (março de 1999). «Homotypic and heterotypic interaction of the neurofibromatosis 2 tumor suppressor protein merlin and the ERM protein ezrin». J. Cell Sci. 112 (6): 895–904. PMID 10036239
- ↑ Gary R, Bretscher A (agosto de 1995). «Ezrin self-association involves binding of an N-terminal domain to a normally masked C-terminal domain that includes the F-actin binding site». Mol. Biol. Cell. 6 (8): 1061–75. PMC 301263
 . PMID 7579708. doi:10.1091/mbc.6.8.1061
. PMID 7579708. doi:10.1091/mbc.6.8.1061 - ↑ Gary R, Bretscher A (novembro de 1993). «Heterotypic and homotypic associations between ezrin and moesin, two putative membrane-cytoskeletal linking proteins». Proc. Natl. Acad. Sci. U.S.A. 90 (22): 10846–50. PMC 47875
 . PMID 8248180. doi:10.1073/pnas.90.22.10846
. PMID 8248180. doi:10.1073/pnas.90.22.10846 - ↑ Gautreau A, Poullet P, Louvard D, Arpin M (junho de 1999). «Ezrin, a plasma membrane-microfilament linker, signals cell survival through the phosphatidylinositol 3-kinase/Akt pathway». Proc. Natl. Acad. Sci. U.S.A. 96 (13): 7300–5. PMC 22080
 . PMID 10377409. doi:10.1073/pnas.96.13.7300
. PMID 10377409. doi:10.1073/pnas.96.13.7300 - ↑ Mykkänen OM, Grönholm M, Rönty M, Lalowski M, Salmikangas P, Suila H, Carpén O (outubro de 2001). «Characterization of human palladin, a microfilament-associated protein». Mol. Biol. Cell. 12 (10): 3060–73. PMC 60155
 . PMID 11598191. doi:10.1091/mbc.12.10.3060
. PMID 11598191. doi:10.1091/mbc.12.10.3060 - ↑ Koltzscher M, Neumann C, König S, Gerke V (junho de 2003). «Ca2+-dependent binding and activation of dormant ezrin by dimeric S100P». Mol. Biol. Cell. 14 (6): 2372–84. PMC 194886
 . PMID 12808036. doi:10.1091/mbc.E02-09-0553
. PMID 12808036. doi:10.1091/mbc.E02-09-0553 - ↑ Granés F, Urena JM, Rocamora N, Vilaró S (abril de 2000). «Ezrin links syndecan-2 to the cytoskeleton». J. Cell Sci. 113 (7): 1267–76. PMID 10704377
- ↑ Brdicková N, Brdicka T, Andera L, Spicka J, Angelisová P, Milgram SL, Horejsí V (outubro de 2001). «Interaction between two adapter proteins, PAG and EBP50: a possible link between membrane rafts and actin cytoskeleton». FEBS Lett. 507 (2): 133–6. PMID 11684085. doi:10.1016/s0014-5793(01)02955-6
- ↑ Reczek D, Berryman M, Bretscher A (outubro de 1997). «Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family». J. Cell Biol. 139 (1): 169–79. PMC 2139813
 . PMID 9314537. doi:10.1083/jcb.139.1.169
. PMID 9314537. doi:10.1083/jcb.139.1.169 - ↑ Yun CH, Lamprecht G, Forster DV, Sidor A (outubro de 1998). «NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin». J. Biol. Chem. 273 (40): 25856–63. PMID 9748260. doi:10.1074/jbc.273.40.25856
- ↑ Sitaraman SV, Wang L, Wong M, Bruewer M, Hobert M, Yun CH, Merlin D, Madara JL (setembro de 2002). «The adenosine 2b receptor is recruited to the plasma membrane and associates with E3KARP and Ezrin upon agonist stimulation». J. Biol. Chem. 277 (36): 33188–95. PMID 12080047. doi:10.1074/jbc.M202522200
- ↑ Barreiro O, Yanez-Mo M, Serrador JM, Montoya MC, Vicente-Manzanares M, Tejedor R, Furthmayr H, Sanchez-Madrid F (junho de 2002). «Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes». J. Cell Biol. 157 (7): 1233–45. PMC 2173557
 . PMID 12082081. doi:10.1083/jcb.200112126
. PMID 12082081. doi:10.1083/jcb.200112126
Leitura adicional
* Martin TA, Harrison G, Mansel RE, Jiang WG (2004). «The role of the CD44/ezrin complex in cancer metastasis.». Crit. Rev. Oncol. Hematol. 46 (2): 165–86. PMID 12711360. doi:10.1016/S1040-8428(02)00172-5
 | Este artigo sobre Genética é um esboço. Você pode ajudar a Wikipédia expandindo-o.
|
 Portal da genética
Portal da genética