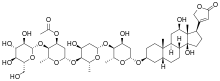
Lanatozid C
 | |||
| Klinički podaci | |||
|---|---|---|---|
| AHFS/Drugs.com | C.html Monografija | ||
| Identifikatori | |||
| CAS broj | 17575-22-3 | ||
| ATC kod | C01AA06 | ||
| PubChem[1][2] | 656630 | ||
| ChemSpider[3] | 570984 | ||
| UNII | 5RR3JFZ771  Y Y | ||
| KEGG[4] | D01972  Y Y | ||
| ChEMBL[5] | CHEMBL506569  Y Y | ||
| Hemijski podaci | |||
| Formula | C49H76O20 | ||
| Mol. masa | 985,116 | ||
| SMILES | eMolekuli & PubHem | ||
| |||
| Sinonimi | [6-[6-[6-[[12,14-dihydroxy- 10,13-dimethyl- 17-(5-oxo-2H-furan-3-yl)- 1,2,3,4,5,6,7,8,9,11,12,15,16,17- tetradecahydrocyclopenta[a]phenanthren- 3-yl]oxy]- 4-hydroxy- 2-methyloxan- 3-yl]oxy- 4-hydroxy- 2-methyloxan-3-yl]oxy- 2-methyl- 3-[3,4,5-trihydroxy- 6-(hydroxymethyl)oxan-2-yl]oxyoxan- 4-yl] acetate | ||
| Farmakoinformacioni podaci | |||
| Trudnoća | ? | ||
| Pravni status | |||
Lanatozid C je organsko jedinjenje, koje sadrži 49 atoma ugljenika i ima molekulsku masu od 985,116 Da.
Osobine
| Osobina | Vrednost |
|---|---|
| Broj akceptora vodonika | 20 |
| Broj donora vodonika | 8 |
| Broj rotacionih veza | 12 |
| Particioni koeficijent[6] (ALogP) | 0,6 |
| Rastvorljivost[7] (logS, log(mol/L)) | -5,5 |
| Polarna površina[8] (PSA, Å2) | 288,3 |
Reference
- ↑ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today 15 (23-24): 1052-7. DOI:10.1016/j.drudis.2010.10.003. PMID 20970519. edit
- ↑ Evan E. Bolton, Yanli Wang, Paul A. Thiessen, Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry 4: 217-241. DOI:10.1016/S1574-1400(08)00012-1.
- ↑ Hettne KM, Williams AJ, van Mulligen EM, Kleinjans J, Tkachenko V, Kors JA. (2010). „Automatic vs. manual curation of a multi-source chemical dictionary: the impact on text mining”. J Cheminform 2 (1): 3. DOI:10.1186/1758-2946-2-3. PMID 20331846. edit
- ↑ Joanne Wixon, Douglas Kell (2000). „Website Review: The Kyoto Encyclopedia of Genes and Genomes — KEGG”. Yeast 17 (1): 48–55. DOI:10.1002/(SICI)1097-0061(200004)17:1<48::AID-YEA2>3.0.CO;2-H.
- ↑ Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B, Overington JP. (2012). „ChEMBL: a large-scale bioactivity database for drug discovery”. Nucleic Acids Res 40 (Database issue): D1100-7. DOI:10.1093/nar/gkr777. PMID 21948594. edit
- ↑ Ghose, A.K., Viswanadhan V.N., and Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A 102: 3762-3772. DOI:10.1021/jp980230o.
- ↑ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE. (2001). „Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”. Chem Inf. Comput. Sci. 41: 1488-1493. DOI:10.1021/ci000392t. PMID 11749573.
- ↑ Ertl P., Rohde B., Selzer P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”. J. Med. Chem. 43: 3714-3717. DOI:10.1021/jm000942e. PMID 11020286.
Literatura
- Hardman JG, Limbird LE, Gilman AG. (2001). Goodman & Gilman's The Pharmacological Basis of Therapeutics (10 izd.). New York: McGraw-Hill. DOI:10.1036/0071422803. ISBN 0-07-135469-7.
- Thomas L. Lemke, David A. Williams, ur. (2007). Foye's Principles of Medicinal Chemistry (6 izd.). Baltimore: Lippincott Willams & Wilkins. ISBN 0-7817-6879-9.
Spoljašnje veze
 | Portal Medicina |
 | Portal Hemija |

Lanatozid C na Wikimedijinoj ostavi
- Lanatoside C





